Water Sanitation Part 3b: Oxidizers Used for Water Sanitation


In a previous article, we discussed common oxidizers that are used for water sanitation. These included chlorine gas, chlorine dioxide, calcium hypochlorite, sodium hypochlorite, ozone and activated peroxygen. In this article, the focus will be on other types of sanitizing systems used for irrigation water, how they work and their advantages and disadvantages. These sanitizing systems include: copper ionization, ultraviolet radiation, heat treatment and slow sand filtration.
Copper Ionization
A copper ionization system consists of two copper electrodes that are positioned parallel to each other inside a pipe. As water flows over these electrodes through the pipe, an electrical current is applied between these electrodes, which displaces copper ions from one of the electrodes. The copper ions are carried away and then they firmly attach to pathogens, disrupting their cell walls, and thereby killing them. Copper ions also attach to the organic matter present in the water. Ideally free copper should be applied at rates of 0.5-1.0 ppm Cu to reduce water-borne pathogens and 1-2 ppm Cu to reduce algae.
The most common oxidizing agents used to clean irrigation water will be discussed in the following chart below. These are chlorine gas, chlorine dioxide, calcium hypochlorite, sodium hypochlorite, ozone and activated peroxygen.

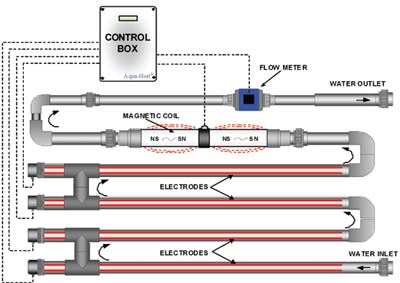
Advantages of copper ionization include: the residual copper in the water can still kill pathogens after water has passed through copper ionization, the system is easy to use, and it is safe for workers. It requires a moderate initial investment, but has low operating costs, which include the electricity to run the system and annual replacement of copper electrodes. It is ideal where water flow rates are high as there is no minimal contact time with water required. Although some copper is bound up in organic matter, it is not as critical as with other sanitation systems, making it acceptable for recycled water. Copper application rates required to control pathogens are low enough that it should not cause phytotoxicity in plants.
As stated, recycled or pond water still needs to be filtered to remove the organic matter that copper ions would bind to. Maintain water pH below 7.0 to keep copper from forming precipitates. The electrical conductivity (E.C.) of the water is important because as it changes, so does the copper output from an electrode. For example, the higher the E.C. of the irrigation water, the more electrical current the water carries, which results in higher release of copper ions. If the water source has changes from well water to rain water, which has zero E.C., then copper ion release is very low and will not control pathogens. It is best to use a copper ionization system that automatically adjusts the copper output based on changes in the E.C. of the water.
Ultraviolet Radiation
Not all types of ultraviolet light are effective at controlling pathogens. However, ultraviolet radiation within the spectrum range of 280-100 nanometers (known as UV-C) is effective against pathogens. Ultraviolet radiation works by altering DNA in the cells of microorganisms, making them incapable of reproducing. Most ultraviolet radiation systems consist of a tubular chamber in which an ultraviolet light bulb is housed inside a quartz tube that runs the length within the center of the chamber. Water goes into one end of the chamber and flows around the outside of the UV light bulb for a set time, sanitizing the water. Once sanitized, the water flows out the other end of the chamber.

Advantages of this system include lack of use of harsh chemicals, no dangerous by-products and it is easy to use. Water temperature, pH and EC have no influence on the efficacy of UV radiation sanitation and there are no known phytotoxicity concerns for plants. Some sources have found that the amount of UV light required to control bacteria is 3.5-26.5 millijoules/cm2 and 6.6-440 millijoules/cm2 for viruses. Some sources recommend that UV light intensity be 100 millijoules/cm2 for recycled water. These levels can be obtained by selecting a bulb with a high UV light output and/or slowing the flow rate of water through the UV chamber to increase the time of exposure. Since there is no residual control from UV radiation, often it is combined with ozone, peroxide or chlorine to add a residual product to the water source in case a pathogen is introduced prior to irrigating a crop.
UV light has to penetrate all portions of the water to sanitize it. If the water is cloudy and/or has particulate matter in it, it causes UV light to disperse, reducing sanitation. Therefore the water has to be filtered to reduce total suspended solids for maximum UV transmission through water. Ideally water should have a turbidity of 2 nephelometric turbidity units (typical unit of measure for turbidity) or less.
UV light systems need some maintenance. The quartz sheath surrounding the UV light bulb has to be dipped in acid every 3-6 months to remove calcium and other mineral deposits that reduce light transmission. Overtime UV bulbs lose effectiveness, so they need to be replaced at least annually and ballasts occasionally burn out.
Heat treatment
This simply means that irrigation water is heated with a heat exchanger to a certain temperature in order to kill pathogens. One suggestion is to raise the water temperature to 203oF (95oC) for 30 seconds. One source found that viruses can be inactivated if the water temperature reaches 158oF (70oC) for 5 minutes. Heat treatment is a simple process that is safe and has no harsh chemicals or by-products. Heat treatment works on water regardless of the water temperature, pH, E.C. or organic debris in the water.

A major disadvantage of heat treatment is that it is one of the most expensive ways to sanitize water. Beyond the high electrical costs, a sizeable holding tank is required in which water is heated to the proper temperature and then the water must cool down before being used to water plants. It would not be practical for high volume water usage as it would take excessive time to heat and then cool water stored in very large storage tanks. There is no residual control for pathogens, so ozone, chlorine, etc. may have to be used if there is concern of recontamination of the sanitized water prior to applying it to a crop. To avoid mineral build-up on the heat exchangers used to heat the water, it is best to drop the water pH to 4.5, but the pH may need to be raised before applying to a crop.
Slow Sand Filtration
There are many different types of media such as screen filters, filter cartridges, sheet filters and disc filters that are used to remove debris and possibly pathogens from irrigation water. At best, these filters screen out larger fungal spores, but slow sand filtration systems can remove much smaller particles including most pathogens, except viruses. A slow sand filter consists of a sand bed, at least 1 meter (1.1 yd) deep, with a large horizontal surface area. Often there is a layer of gravel below the sand layer and a perforated pipe to collect the water. Water goes into the top and gravimetrically flows through the sand and then exits out the bottom where it is stored in a holding tank. In the top 6 inches (15 cm) of the sand filter, a biofilm layer forms, known as “Schmutzdecke”, which filters out most of the pathogens and other fine particles. Some sand filter systems will force water through a sand filter at faster rates, but are not effective at pathogen removal.
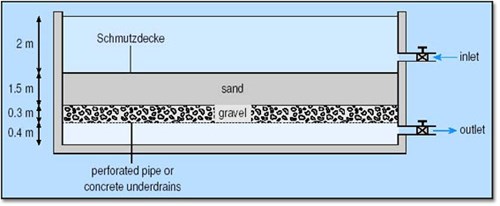
Most slow sand filtration systems have an average flow rate of 2-4 gallons/min/yd2 (9.1-18 liters/min/m2) and yield about 76,000 gallons (288 m3) of water per day per 67 yd2 (80 m2) of sand filter surface area. Upfront costs are high for the sand, housing to hold it and a holding tank for the water, but there is little to no operating costs other than the electricity to pump the water into the filter and the occasional raking of the surface biofilm layer that overtime can clog the filter. The filter must stay continuously wet to keep the biofilm layer alive. This is accomplished by keeping a constant layer of water on top of the sand which also serves to place hydraulic pressure on the system to encourage water flow through the filter. The filter surface cannot be disturbed (unless it must be raked). Slow sand filtration may not produce sufficient capacity for peak water demands and may not eliminate all pathogens. There is no residual control, so secondary water sanitation may be needed.
References:
- Powell, C.C. 2001. "Are Your Plants Drinking Dirty Water?" Grower Talks 79(7)
- http://pnwhandbooks.org/plantdisease/pesticide-articles/treating-irrigation-water-eliminate-water-molds
- Newman, S.E. 2004. "Disinfecting Irrigation Water for Disease Management." 20th Annual Conference on Pest Management on Ornamentals – Society of American Florist Meeting (San Jose, CA)
- Harris, M.A and L.R. Oki. 2009. "Using Slow Sand Filters to Remove Plant Pathogens from Irrigation Runoff." Presentation at International Plant Propagators’ Society Meeting in San Diego, CA October 2, 2009