Sampling Procedures for Testing Water and Fertilizer Solution


Testing water and fertilizer is important as they directly influence the pH and nutrient levels in the growing medium. As a result, they both have a significant impact on crop quality. It is important to periodically test the water to verify that the fertilizer you are using is appropriate for your water and if acid injection is sufficient or needed. The fertilizer solution analysis verifies that the fertilizer you are using is formulated correctly and also indicates the application rate. Here are more details on why testing the water and fertilizer is important and how to collect the samples.
Why Test Your Water?
Testing your water is the single most important test. Consider that the alkalinity of the water (composed mostly of bicarbonates and carbonates) directly affects the pH of the growing medium. Knowing the alkalinity makes it easy to select the proper fertilizer. It makes it possible to determine the fertilizing solution that can offset the pH-changing influence of the water alkalinity and to determine if an acid injection is necessary and at which rate. The water may also provide sufficient beneficial nutrients such as calcium, magnesium and sulfate, which are often missing from most fertilizers. A disadvantage is that water can also provide high levels of waste elements such as chloride, fluoride and sodium, which can interfere with plant uptake of fertilizer elements. Lastly, water also contains dissolved salts, measured as electrical conductivity (E.C.). High salt contents may require the frequent leaching of the growing medium or the treatment of the water with a reverse osmosis unit.

How to Collect the Water Sample?
When collecting a water sample for testing, it should be taken from the same source that is applied to the crop. If acid is injected, it is best to test the raw water and also the acidified water for comparison. If more than one water source is used to irrigate your crops, then each source should be tested separately.
Water should be collected from the spigot or hose end (make sure to flush out stagnant water, fertilizer residues and chemical residues from the hose before taking the sample). If unsure, run the water for 2 minutes.
Fill up a clean plastic bottle completely, leaving no air space. Air left in the bottle can alter the pH of the water and have a slight influence on nutrient levels. Most laboratories require anywhere from 4-16 ounces of water for testing. Contact the laboratory and ask for a water test kit. Most laboratories provide the kit free of charge and it includes the appropriate size bottle.
Once you receive the results of the water test from the laboratory, please refer to the Grower Services portion of our website or contact your Premier Tech Grower Services Representative for help interpreting the results.
Why Analyze Your Fertilizer Solution?
A fertilizer solution should be tested several times per growing season, especially if multiple fertilizers are used. The fertilizer solution can be tested using two different methods: sending a sample off to a laboratory or in-house testing with an electrical conductivity (E.C.) meter. Laboratory testing determines the amount of each fertilizer element in the fertilizer solution and the application rate. This is helpful if you receive pre-mixed fertilizers to verify whether they were properly blended. In-house testing with an E.C. meter can be used to test the fertilizer application rate coming out of the hose. In-house testing should be done every time a new batch of fertilizer concentrate is mixed.

How to Collect Fertilizer Solution Sample for Laboratory Testing?
Collecting a fertilizer solution sample is similar to collecting a water sample. The fertilizer solution should be collected from the spigot or hose end (make sure to flush out stagnant water, fertilizer residues and chemical residues from the hose before taking the sample). It is best to run the irrigation system for 2 minutes to be sure that the fertilizer sample is blended thoroughly. Fill a clean plastic, leaving a little air space. Contact your laboratory and ask for a fertilizer solution analysis test kit. Again, most laboratories require anywhere from 4-16 ounces of fertilizer solution for testing.
It is best to test every new batch of fertilizer with different analyses. This is to verify that the fertilizer formula posted on the packaging closely matches what the fertilizer solution analysis test indicates. This is also very helpful if you blend individual fertilizer elements to make your own fertilizer. Keep in mind that when injecting fertilizer into the water line, it may not mix uniformly within the water. If so, collect the fertilizer solution coming out the end of the hose in a bucket, mix it and then take the sample.
When you receive the results of a fertilizer solution analysis, note that the designations for phosphorus and potassium in the analysis are not the same as the designation on the fertilizer packaging. Phosphorus is tested as “P”, but is listed on the fertilizer bag as P2O5; likewise, potassium is tested as “K”, but is listed on the fertilizer bag as K2O (see chart below). Total nitrogen as listed on the fertilizer packaging is the summation of ammonium, nitrate and urea. Ammonium and nitrate are tested in the same form as listed on the fertilizer packaging. However, urea is not tested unless specifically requested. All other fertilizer elements as seen on the fertilizer solution analysis are expressed the same as on the fertilizer packaging, with the possible exception of sulfate.
| Element | Element as tested by laboratory | Element as listed on fertilizer packaging | To convert lab value to fertilizer packaging value, multiply by... |
|---|---|---|---|
| Nitrate | NO3 | O3 | - |
| Ammonium | NH4 | NH4 | - |
| Phosphorus | P | P2O5 | 2.33 |
| Potassium | K | K2O | 1.20 |
| Calcium | Ca | Ca | - |
| Magnesium | Mg | Mg | - |
| Sulfate | S | SO4 | 3.00 |
| Iron | Fe | Fe | - |
| Manganese | Mn | Mn | - |
| Copper | Cu | Cu | - |
| Boron | B | B | - |
| Zinc | Zn | Zn | - |
| Molybdenum | Mo | Mo | - |
Using the conversion factors from the chart above, let’s use an example to show how to cross-reference laboratory analyses with the guaranteed analyses on the fertilizer bag. Let’s say that we want to verify that 20-10-20 was mixed properly by the manufacturer. A sample of the 20-10-20 fertilizer solution is sent to a laboratory and the analysis shows the results in the table below. Let’s convert the laboratory numbers into the numbers represented on the fertilizer packaging.
| Element | Analysis result (parts per million - ppm) | Conversion factor | Comparison to fertilizer analysis on packaging |
|---|---|---|---|
| Nitrate | 60 ppm | - | 60 ppm |
| Ammonium | 40 ppm | - | 40 ppm |
| Phosphorus | 21.8 ppm | 2.33 | 50 ppm |
| Potassium | 83 ppm | 1.20 | 100 ppm |
If we add nitrate and ammonium values together, it is equal to 100 ppm nitrogen (60 ppm + 40 ppm), which is also the fertilizer application rate. If we take the ratio N-P-K, then the ratio using the above numbers is 100-50-100. Divide each of the numbers by 5 and we have a ratio of 20-10-20, which is an accurate analysis of the fertilizer. Therefore, this fertilizer was correctly made by the manufacturer and it is being applied at 100 ppm nitrogen. If the fertilizer contains urea, it is also part of the total nitrogen on the fertilizer packaging and it must be taken into consideration when determining the fertilizer application rate.
How to Collect Fertilizer Solution Sample for In-house Testing?
In-house testing of the fertilizer solution with an E.C. meter can be used to verify the fertilizer application rate; it will not provide the levels of each nutrient. To collect a fertilizer solution sample, follow the same procedure as above.
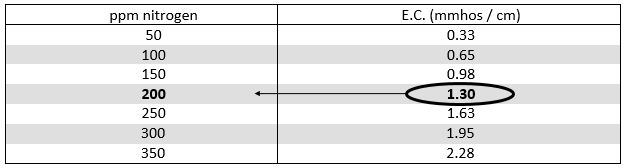
To determine the fertilizer application rate, start by calibrating the E.C. meter with a conductivity standard solution. Then test the E.C. of the fertilizer solution coming out the end of the hose. Next, test the E.C. of the water and record the value. Subtract the water E.C. from the fertilizer solution E.C. and this will provide the E.C. of the fertilizer only. Refer to the fertilizer packaging or the manufacturer’s website that can cross-reference fertilizer E.C. to ppm nitrogen (see example below).
Example: How much 20-10-20 is being applied as tested below?
E.C. of fertilizer solution = 2.10 mmhos/cm
(minus) - E.C. of water = 0.80 mmhos/cm
E.C. of added fertilizer = 1.30 mmhos/cm (2.10 - 0.80)
Answer: 200 ppm Nitrogen
If the fertilizer application rate coming out the end of the hose is not correct, remember that the fertilizer concentrate may have been improperly mixed, the injector may be set at the wrong ratio or the fertilizer injector needs maintenance.
To find out more about how to take growing media and tissue samples for laboratory analysis, see our article Media and Tissue Testing - Media Testing Methods on our Training Center.