Relationship Between Fertility Extremes and Growing Medium pH


The pH of a growing medium increases or decreases depending on the fertilizer used, the crop grown, and the water alkalinity. However, pH change is usually gradual. When fertility levels in the growing medium are either very low or excessive, the pH of the growing medium can change rapidly. When the fertilizer level is very low in the growing medium (measured as electrical conductivity (E.C.)), there is a rapid increase in the growing medium's pH. Likewise, when the E.C. of a growing medium becomes excessive because of a potentially acidic fertilizer, the pH drops. So why does this happen and is it a concern for the crop grown?
Low Fertility = Higher pH?
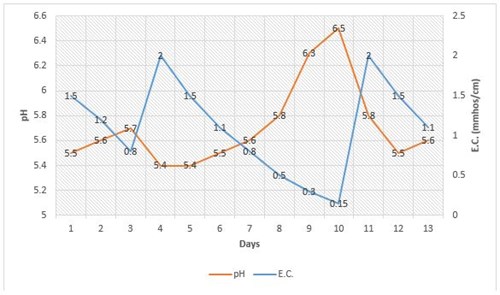
Typically, when a crop is grown in a growing medium where the overall fertility level is very low (E.C. is below 0.3 mmhos/cm), the pH tends to increase rapidly by approximately 0.5 pH unit. This can be seen in Figure 1 on days 8-10, when the pH rose from 5.8 to 6.5 as the E.C. dropped below 0.3 mmhos/cm. However, if the same growing medium with the same crop is fertilized more frequently so the E.C. does not drop below 0.5 mmhos/cm, the pH changes very little. The phenomenon of sudden pH climb due to low E.C. often occurs when growers feed at very low rates, withhold fertilizer to minimize plant growth or simply do not feed at all. It is believed that this occurs because the plant has few fertilizer elements to take up from the growing medium that can generate acid through the roots. In addition, the water alkalinity and limestone in the growing medium work together to also increase the pH of the growing medium.

The good news is that this 0.5 pH unit increase is “artificial” and as seen in Figure 1, the pH quickly dropped back down to 5.8 by simply applying the recommended rate of a potentially acidic fertilizer, such as 20-10-20. Because the pH of the growing medium responded so rapidly when fertility levels were brought back up to normal, the “real pH” of the growing medium was not 6.5 or 6.3, but about 5.8. Although the pH was high, it is not a concern if fertilizer is applied at normal rates. However, if fertilizer is not applied for more than a week, then the high pH and very low fertilizer levels will cause nutrient deficiencies.
Excess Fertility = Lower pH?
Just as very low E.C. leads to a rapid pH increase in a crop, excessive fertility levels from a potentially acidic fertilizer or even a potentially basic fertilizer can “artificially” cause a rapid pH drop of about 0.5 mmhos/cm. This can be seen in Figure 2 on days 4 and 9. This pH drop can quickly be corrected simply by allowing the plant to take up the excess fertilizer, thereby bringing the E.C. down to a normal level of less than 2.5 mmhos/cm (as seen in Figure 2, days 5-8), or by leaching (Figure 2, day 10). It makes sense when applying a potentially acidic fertilizer that the pH may decline depending on the water alkalinity. However, when E.C. exceeds 3.5 mmhos/cm, there seems to be a rapid drop in pH that can quickly be reversed when the E.C. in the growing medium is brought down to normal levels.
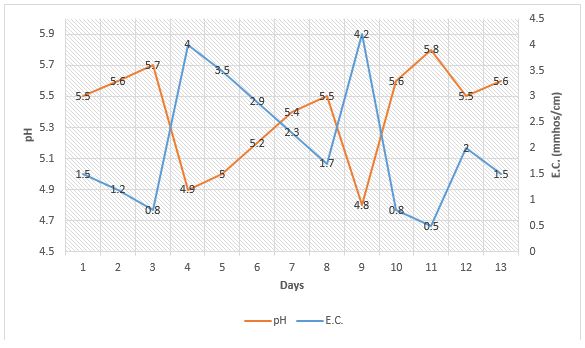

Fertilizer salts can become excessive in the growing medium from applying excessive levels of water soluble fertilizer at one time, accumulation of fertilizer in the growing medium from overfeeding, or from high application rates of controlled release fertilizer. In the last example, most controlled release fertilizers are acidic. If they are applied at high rates or it becomes hot, it is typical that fertilizer levels become excessive in the growing medium and pH drops.