MYTH Series: Water pH Relates to Medium pH

The pH measures the hydrogen ions (H+) and the hydroxide ions (OH-) in a solution such as water. The range of pH is from ‘0’ (very acidic: the concentration of H+ ions is higher than the concentration of OH-) to ‘14’ (very basic: the concentration of OH- ions is higher than the concentration of H+). When pH is 7, it is neutral, which means the concentration of H+ and OH- is the same. The pH of the water is important when it comes to mixing with pesticides and dissolving micronutrients in water. However, the water pH has little influence on the pH of the growing medium. As discussed below, other factors impact the pH of the growing medium.
Water Alkalinity
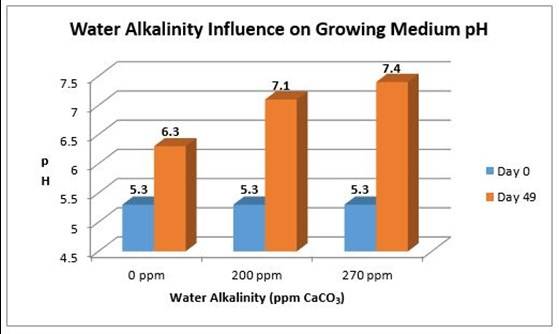
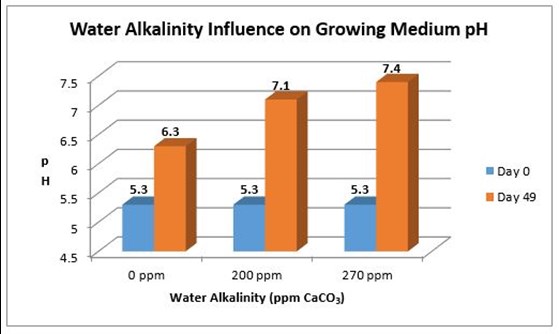
The pH of the irrigation water is not as important as its alkalinity (CaCO3) when dealing with non-inert growing media, such as peat moss. Alkalinity measures the amount of carbonates and/or bicarbonates, or the amount of limestone dissolved in the irrigation water. When water has high alkalinity, it means that the water has high limestone. More limestone is added to the growing medium every time watering occurs, increasing its pH over time (Figure 1). In addition, water with high alkalinity has a larger buffer capacity than water with low alkalinity. "Buffer capacity" refers to the ability to resist change; therefore, the higher the buffer capacity, the greater the resistance to change and the ability to lower the pH of the water.
There are ranges for the optimum alkalinity for different container sizes:
- Plugs: 60-100 ppm CaCO3
- Small pots (4” and smaller), bedding plant flats: 80-140 ppm CaCO3
- Large pots (>4”), long-term crops: 120-180 ppm CaCO3
The main difference between these ranges is the relation to the fertilizer application rate and the size of the plant for each container size. The effect of an acidic fertilizer in a small plug is going to be too little since the rate would be low and the plant is very small compared to the rate of a large pot (see discussion under fertilizer title).
Let’s think about a plug grower who is growing in the same peat-based growing medium in two different geographical locations. In both cases this grower is watering with well water with a pH of 8. The water alkalinity at location #1 is 300 ppm CaCO3 and 150 ppm CaCO3 for location #2. Based on the normal range of 60-100 ppm CaCO3, we can conclude that the alkalinity of both water sources is high and acid needs to be injected to drop alkalinity; for this case 80 ppm CaCO3 will be the target. The results are shown in Table 1:
| Location | Starting pH | Water Alkalinity (ppm CaCO3) | fl oz/gallon Sulfuric Acid* to Reduce the Alkalinity to 80 ppm | Final pH |
|---|---|---|---|---|
| 1 | 8 | 300 | 6.27 | 5.9 |
| 2 | 8 | 150 | 2.02 | 6.4 |
Notice that the amount of sulfuric acid needed to drop the alkalinity to 80 ppm CaCO3 and subsequent pH was based on the alkalinity of the water, not the pH of the water.
Fertilizer
As plants take up fertilizer, they emit hydrogen and hydroxyl/bicarbonate ions into the growing medium, effectively changing the growing medium pH. Generally the larger the plant, the more fertilizer is used and therefore the faster the plant can change the pH of the growing medium. All fertilizer elements, especially nitrogen, have the effect of either lowering or raising growing medium pH. Nitrogen is available in three different forms: nitrate, ammonium and urea. Nitrate (NO3-) has a negative charge, ammonium (NH4+) has a positive charge and urea (CO(NH2)2) has no charge at all. When plant roots take up nitrate (negatively charged), they release OH- or bicarbonate (HCO3-) to maintain electrical balance. When plants take up ammonium (positively charged), the roots release H+ to balance the charges within the plant. And finally, urea has to be transformed into ammonium to be taken up by the plant. Urea could be further transformed into ammonia and then into nitrate with the help of bacteria. The process of transforming ammonium into nitrate is called nitrification.
Fertilizer with high ammoniacal nitrogen, like 20-20-20, has the potential acidity to neutralize 576 lb of calcium carbonate for each ton of fertilizer absorbed by a crop, or in other words, to lower the pH of the growing medium. Fertilizer with high nitrate nitrogen, like 15-5-15 Cal-Mag, has the effect of adding 131 lb of calcium carbonate (limestone) for each ton of fertilizer used by the plant, or in other words, to increase the pH of the growing medium. By selecting potentially acidic and basic fertilizers based on the type of nitrogen source, a growing medium pH can be maintained within optimum ranges.
It is extremely important to monitor fertilization management when using water with low alkalinity (<40 ppm CaCO3) to water plants. Water with low alkalinity has a limited buffering capacity; therefore, the application of potentially acidic fertilizers can have a huge impact on the pH of the growing medium. In this case, potentially acidic fertilizers can drop growing medium pH to unacceptable levels. The same principle applies to reverse osmosis (RO) water since the alkalinity of the treated water can be extremely low.
The pH of the growing medium is important because it determines the availability of the nutrients. For example, the availability of micronutrients like iron, manganese, zinc and copper increases when the pH is below 5.5. On the other hand, the availability of molybdenum, calcium and magnesium decreases. The opposite occurs at pH higher than 6.5. Based on these facts, we recommend maintaining the pH of the growing medium between 5.5 and 6.5.
Plant Species
Although not as significant, different plant species also influence the pH of the growing medium. For example, zinnia and vinca tend to increase the pH of the growing medium over time, pansies do not have any effect on the pH of the growing medium, and tomato, dianthus and celosia tend to lower the pH of the growing medium.
In conclusion, it is very important to verify the alkalinity of the water before the beginning of each season. By knowing the alkalinity of the water, a grower can make a better decision regarding fertilizer management to maintain the pH of the growing medium within optimum ranges.