How to Calibrate Fertilizer Injectors

Plants require essential elements to maximize their genetic potential. These elements are supplied by air, water, substrates and fertilizers. For water-soluble fertilizers, it is important to make sure your fertilizer injector is working properly and that it is calibrated for accuracy.
About 90% of the weight of the plant is water and the remaining 10% constitutes the dry weight of the essential elements. They are called essential elements because plants cannot grow properly without one of them. There are three non-mineral elements: Carbon (C), Hydrogen (H) and Oxygen (O); they constitute 90% of the plant’s dry weight and are obtained from water and air. In addition, there are 14 mineral elements: Nitrogen (N), Phosphorus (P), Potassium (K), Calcium (Ca), Magnesium (Mg), Sulphur (S), Iron (Fe), Manganese (Mn), Boron (B), Zinc (Zn), Copper (Cu), Nickel (Ni) and Chloride (Cl); those are primarily provided by the fertilizer, but they can also come from water and the substrate. Moreover, they are divided into macronutrients (plants require these elements in high amounts) and micronutrients (plants require these elements in low amounts).
In the greenhouse and nursery industry, most of the mineral nutrients come from water-soluble fertilizers. It is very important to know the actual injection ratio and if the injector is working properly. If the injector is not working properly, nutrient deficiencies or toxicities will likely occur. Several companies sell injectors (Dosatron, DosMatic, Anderson, Smith and others); each company has different injection technologies, but the result is similar from one to the other.
There are different models with different minimum and maximum water flows and injection rates. For example, a small grower would need a medium injection rate (1:50 to 1:500) with a low water flow (0.5 to 20 gpm).

Understanding the Injection Ratio
The injection rate can be expressed as a ratio (1:50, 1:100, 1:200, etc.) or as a percentage (2%, 1%, 0.5%, etc.). The equivalence between those two are 1:50 = (1 unit/50 units)*100 = 2%; 1:100 = (1 unit/100 units)*100 = 1%; and 1:200 = (1 unit/200 units)*100 = 0.5%. The ratio of 1:50 means that there is one unit of stock solution injected into fifty units of water. A fertilizer stock solution that is injected at a ratio of 1:50 is more concentrated coming out the end of the hose than the same stock solution injected at 1:200. In contrast, if a grower is applying a final concentration of 200 ppm of N using an injection ratio of 1:200, and then decides to change the injection ratio to 1:100, the final fertilizer solution would be twice as concentrated or 400 ppm N.

Keep in mind that each fertilizer has a maximum solubility and that it is affected by water temperature (low temperature, low solubility). If fertilizer is added beyond its maximum solubility, it will slow the dissolution process and precipitates will form in the stock tank. Also, some fertilizers cannot be mixed in the same stock solution container (sulfates and calcium, phosphates and calcium, and phosphates and iron) since they will react forming precipitates in the bottom of the stock tank. Finally, stir, stir and stir until all the fertilizer is completely dissolved and stir every half hour while watering plants.
There are two methods to verify the proper operation of injectors. Before starting with the procedure, it is necessary to calibrate scales for weight, get a graduated cup in millilitres (or a beaker), get a 20 L (5 gal) bucket and the fertilizers that are used. The first method consists of calculating the actual injection ratio, and the second method estimates the EC of the fertilizer solution.
Actual Injection Ratio Method:
- Place the suction tube from the injector into a measuring cup or beaker and fill it up with stock solution to a known level.
- Turn on the irrigation system and collect the fertilizer solution in the 20-litre bucket.
- Stop the irrigation system once you have obtained 20 litres of fertilizer solution.
- Calculate the amount of stock solution depleted from the measuring cup and the volume of fertilizer solution in the 20-litre bucket.
Example of Injection Ratio Calculations
Measuring the Injector’s Injection Ratio
A grower is using an injector to fertigate plants; the desirable ratio is 1:100. He/She wanted to know the actual injection ratio; thus, he/she followed the above procedure. The grower collected 20 litres of fertilizer solution. The measuring cup at the beginning of the experiment was 0.50 litre and at the end was 0.32 litre.
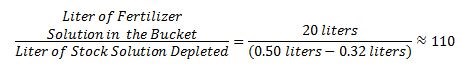
The actual injection ratio is 1:110, not 1:100. Therefore, he/she is not feeding the plants at the desired rate; the fertilizer solution is less concentrated than it is supposed to be. Now, it is necessary to add more fertilizer to the stock solution to account for the injection ratio.
Determining How Much Fertilizer to Add to Stock Solution
Now that the injector has been calibrated, let’s determine how much fertilizer to add to a stock tank to achieve the desired application rate. The variables are:
Application rate of 150 ppm nitrogen
Injector’s actual injection ratio is 1:110
Fertilizer is 20-20-20 or 20% N
Stock tank size: 30 gallons
The formula needed to calculate the amount of fertilizer to add to the stock tank is:

Thus, to make 30 gallons of stock solution, it is necessary to mix 20.6 pounds of 20-20-20 fertilizer.
*Other conversion factors used for different units:
Desired result in ounces per US gallon, use conversion factor of 75 (as above)
Desired result in pounds per US gallon, use conversion factor of 1,200
Desired result in grams per litre, use conversion factor of 10
The EC Method
This method consists of measuring the electrical conductivity (EC) of the fertilizer solution. To start, find out your injector’s injection ratio and the nitrogen concentration. Then, look up the EC of the fertilizer applied. All fertilizers come with a table illustrating these variables. For example, Peter’s 20-10-20 has an EC of 1.24 dS/m (or mmhos/cm) at the injection rate of 1:100 and at 200 ppm of N.
Example of EC Method
To check the proper use of the injector, measure the EC of the water source before adding fertilizer. Next, collect 0.1 litre from the stock solution and mix it with 10 litres of water; in other words, it is a ratio of 1:100. If the nutrient stock solution was properly mixed to provide 200 ppm of N from 20-10-20, the result should be 1.24 dS/m plus the EC from the water source. If the water source has an EC of 0.5 dS/m, the EC of the final fertilizer solution should be 1.74 dS/m. If it is more or less than that, it is necessary to calculate the difference as a percentage and then add or subtract that percentage from the weight of the fertilizer added to the stock tank.
If the result is different from what was expected, the problem could be:
- The stock solution was mixed improperly; it is either more concentrated or less concentrated.
- The injector could be drawing too little or too much solution from the stock tank.
It is required that you calibrate the injector before each season starts and that you check the EC of the fertilizer solution at least once a month during the crop season. For both methods, it is necessary to bleed the injectors before calibrating them to get rid of all the air inside. Also, it is necessary to eliminate all the air from the suction tube (flush it). Finally, these tests have to be done at least three times to confirm a tendency (if it is good, no more actions need to be taken; if it is bad, further actions need to be taken). Keep in mind fertilizers are highly corrosive and that there will probably be some wear and tear on the injectors, year after year. All injectors need maintenance. Do not wait until there is a problem with plant nutrition to calibrate them.
Conversions
3.78 litres = 1 gallon
1 litre = 1,000 millilitres
1 kilogram = 35.27 oz
1 kilogram = 1,000 grams
1 kilogram = 2.2 pounds